Synthesis, Structure and Characterization of Sr0.9Y0.1Ti1-xVxO3 (x = 0.1, 0.2) Anodes for Solid Oxide Fuel Cell
DOI:
https://doi.org/10.37934/armne.19.1.107114Keywords:
Anode materials, solid oxide fuel cell, renewable energy, conductivity, energy materialsAbstract
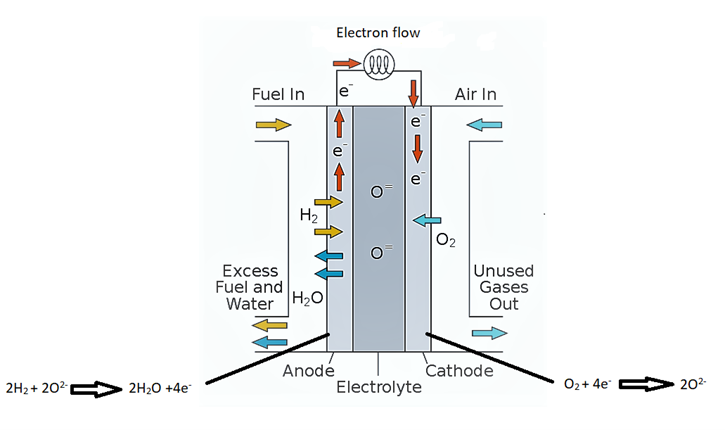
The current anode materials used in solid oxide fuel cells face challenges regarding their thermal stability and performance under varying operating conditions with the commonly used electrolyte (yttria stabilized zirconia), prompting the need for the development of alternative anode materials with superior thermal compatibility. Sr0.9Y0.1Ti1-xVxO3(x=0.1, 0.2) was synthesized via the traditional solid-state reaction method and characterized as potential anode materials for SOFCs. The crystal structure, phase purity, electrical conductivity, and microstructure of the synthesized materials were analyzed using X-ray diffraction (XRD), scanning electron microscopy (SEM), energy-dispersive X-ray spectroscopy (EDS), thermogravimetric analysis (TGA), and conventional four-probe techniques. Results obtained from the experimental investigations showed a negligible weight loss in Sr0.9Y0.1Ti1-xVxO3(x=0.1, 0.2) samples of about 18.81%, and a 2.2% weight gain for x = 0.2. The negligible weight loss indicates that Sr0.9Y0.1Ti1-xVxO3(x=0.1, 0.2) would be a highly stable material under the high operating temperature of SOFC. At 900 °C, the electrical conductivity in air for Sr0.9Y0.1Ti1-xVxO3(x=0.1, 0.2) was measured to be 0.32 Scm-1 and 0.2042 Scm-1, respectively. The result of linear thermal elongation investigation revealed that Sr0.9Y0.1Ti1-xVxO3(x=0.1, 0.2) has a favourable thermal expansion coefficient. This will help reduce the challenges of thermal compatibility associated with commonly used electrolytes in SOFC.