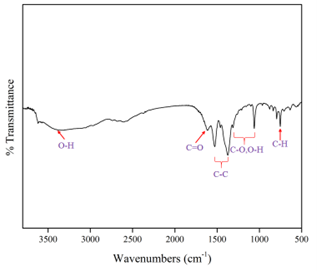
Unary Adsorption Isotherm of Carbon Dioxide and Methane of Nickel Gallate Metal-organic Framework
DOI:
https://doi.org/10.37934/armne.19.1.5162Keywords:
Adsorption, MOF, isotherm, thermodynamic, kineticAbstract
Carbon dioxide (CO2) and methane (CH4) adsorption using metal-organic frameworks (MOFs) has garnered continuous interest in academia and industrial fields due to their remarkable performances. A series of studies have been dedicated to design the potential MOFs for CO2 and CH4 adsorption. Among them, nickel gallate MOF (Ni-gallate) can act as a promising adsorbent based on the predicted adsorption capacity and selectivity. However, the behaviors of CO2 and CH4 unary adsorption isotherms of Ni-gallate have not been studied by isotherm and kinetic models. Therefore, the aim of this work is to perform pure CO2 and CH4 gas adsorption and the unary adsorption isotherms were explained by the isotherm and kinetic models. Based on the unary adsorption isotherms, CO2 adsorption capacity values were observed to be higher than CH4 which resulted in great selectivity values. For isotherm models, the Toth model demonstrated the highest goodness-of-fit compared to Langmuir, Freundlich and Sips models and the thermodynamic properties were determined using its constant values. On the other hand, the kinetic data was best-fitted by the pseudo-first order model compared to the pseudo-second order and Elovich models and its constant values were used to determine activation energy. This useful information is very important for the design and operation of CO2 and CH4 adsorption systems.