Synthesis of Zeolite by Using Waste Cans as a Source of Aluminium and Testing its Performance as a Dye Adsorbent
DOI:
https://doi.org/10.37934/armne.19.1.3850Keywords:
Adsorbent, textile dye, waste cans, waterglass, zeoliteAbstract
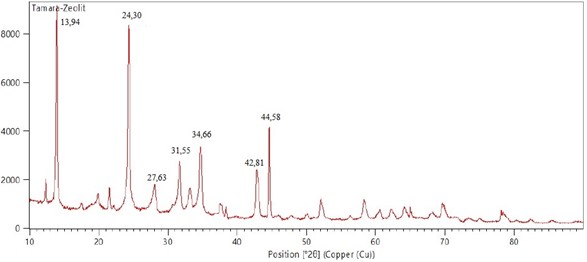
Aluminium metal has corrosion-resistant properties, light and easy to obtain, so it is widely used as a raw material for making soft drink cans. The aluminium content in used cans can be more than 90% and it can be used as raw material for making zeolite. In this study, zeolite was synthesized, and its performance was tested as an adsorbent of water-soluble (Congo red) and water-insoluble (Naphthol blue black) textile dye waste. The method used in this study includes three stages, namely (1) synthesis of zeolite from waste cans as a source of Al and waterglass as a source of Si, (2) characterization of zeolite produced using XRD, FTIR, and SEM, and (3) performance test of zeolite on Congo red and Naphthol blue black dyes with adsorbent weight variation: 0.1 to 0.5 gram in 10 mL of a prepared sample at a concentration of 20 mg/L; contact time variations: 30 to 120 minutes at a speed of 150 rpm; pH variations: 5, 7, and 9; and variations in the initial concentrations of dyes of 10 to 40 ppm. The decrease in dye concentration was measured using a UV-Vis spectrophotometer. The Al content in the used cans obtained is 96.5%. The zeolite was successfully synthesized, and the yield obtained from the zeolite formation reaction was 92.67%. XRD characterization results show the diffraction pattern of a mixture of zeolite-X and zeolite-NaP1 with an average crystal size of 24,08 nm. The purity of the synthetic zeolite for zeolite X was 55.44% and zeolite Na-P1 was 44.53%. FTIR characterization shows the intensity of functional groups T-O, T-O-T or O-T-O, and T-H, where T is Si or Al. Characterization by SEM, synthetic zeolite has the same morphology as Faujasite zeolite. The maximum adsorption capacity of zeolite on Congo red and Naphthol blue black was 321,2921 mg/g and 77,5354 mg/g based on Freundlich isotherm. The optimum conditions for adsorption of Congo red and Naphthol blue black dye were at 0,4 gram and 0.1 gram of adsorbent weight, 30 minutes of contact time, 5 and 7 of pH and 40 mg/L initial concentrations of dyes.