Heat of Combustion and Thermal Decomposition of Ammonium Perchlorate/Sorbitol Solid Propellant with Metal Additives
DOI:
https://doi.org/10.37934/arefmht.18.1.94105Keywords:
Ammonium perchlorate, sorbitol, specific impulse, thermal decomposition, ignition temperature, catalystAbstract
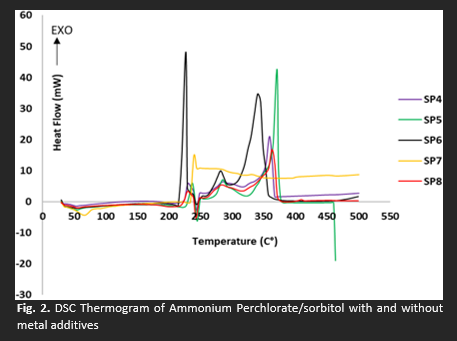
Ammonium perchlorate (AP) stands out as the predominant oxidizer in solid rocket propulsion systems, while sorbitol, frequently employed as a fuel in sounding rockets, is expected to generate non-hazardous gases during combustion. This study reports on the effect of sorbitol as potential organic green fuel in addition to additive metals on heat of combustion, thermal properties and specific impulse for ammonium perchlorate based solid propellant. The specific impulse was measured using PROPEP 3.0 (Propellant evaluation program). Solid propellant characterization included measuring energy using a bomb calorimeter and conducting thermal analysis using DSC and TGA. Various compositions were prepared by varying the AP and sorbitol content in the propellant, and additive metals like magnesium and ferrite were introduced. The findings reveal that the formulation of 77% ammonium perchlorate into the 23 % sorbitol releases the optimum combustion energy at 2144.47 KJ/mol and lowers the thermal decomposition temperature of AP to 210.38 °C compared to common AP solid propellant formulation. Meanwhile, the addition of 1 % magnesium metal to the mixture significantly improves the combustion energy, propellant performance, and decreases thermal decomposition temperature of the AP/sorbitol. The addition of 0.05% ferrous oxide shows a catalytic capability to decrease the ignition temperature of the sample to 199.15 °C and merge two exothermic peaks into single peak. The main outcome of this work is, AP can interact synergistically with sorbitol mixed with both additive metals by increase the heat combustion, specific impulse, improve AP sensitivity toward heat and lower the thermal decomposition temperature.Downloads
Download data is not yet available.
Downloads
Published
2025-01-17
How to Cite
Azizi, M. Z. ., Abdul Hamid, A. H. ., Salleh, Z. ., Abdul Ghaffar, Z. ., Adnan, A. A. ., Yaacob, I. N. ., Salleh, N. ., & Pardorla, W. J. K. (2025). Heat of Combustion and Thermal Decomposition of Ammonium Perchlorate/Sorbitol Solid Propellant with Metal Additives. Journal of Advanced Research in Experimental Fluid Mechanics and Heat Transfer, 18(1), 94–105. https://doi.org/10.37934/arefmht.18.1.94105
Issue
Section
Articles