Iron Oxide Nanoparticles Derived from Chlorella Vulgaris Extract: Characterization and Crystal Violet Photodegradation Studies
DOI:
https://doi.org/10.37934/progee.24.1.110Keywords:
Crystal violet, Iron oxide nanoparticle, Photodegradation, Microalgae, ExtractAbstract
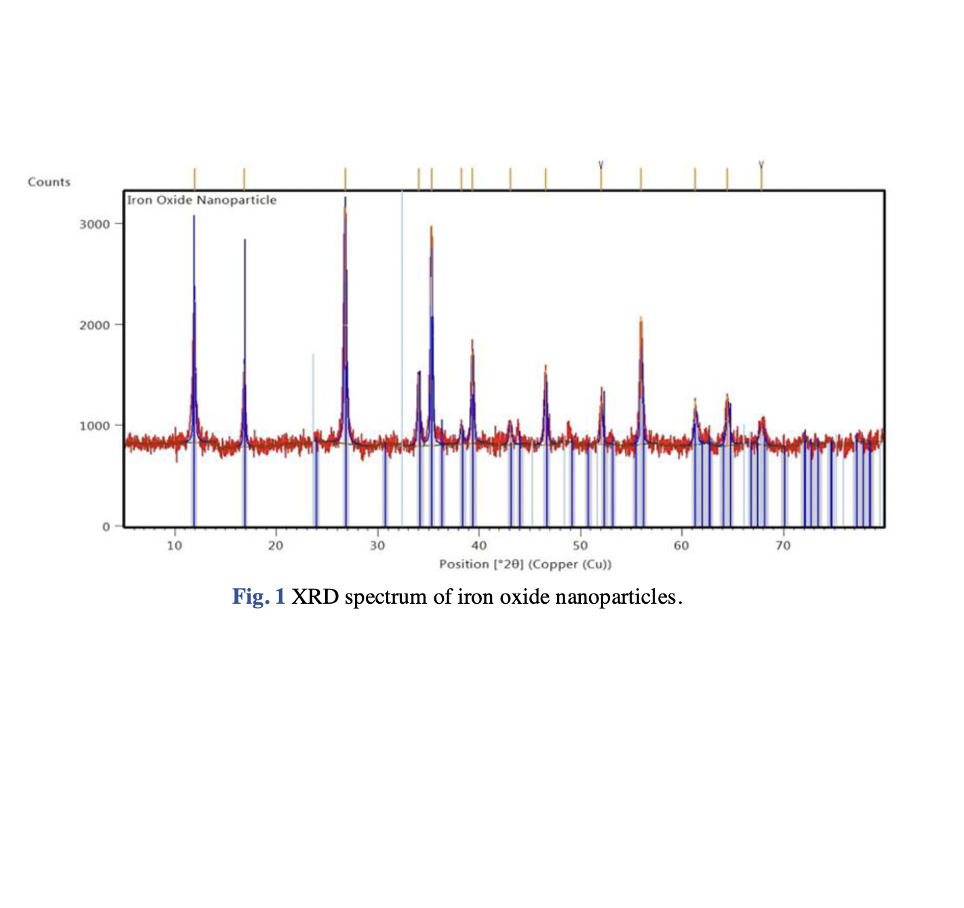
Nanoparticles were first used a century ago, but have recently gained popularity due to their ease of use, eco-friendliness, pollution-free nature, nontoxicity and low cost for wastewater treatment applications. In terms of nanoparticles preparation, green synthesis is a more convenient, economical, quick, and environmentally friendly process than traditional synthesis (i.e. chemical and mechanical) methods. The objective of this study was to synthesise iron oxide nanoparticles from iron (III) chloride using microalgae (Chlorella vulgaris) extract for photodegradation of crystal violet (CV) dye. Various characterization methods such as X-ray diffraction (XRD), field emission scanning electron microscopy (FESEM), and Fourier-transform infrared spectroscopy (FTIR) were used to examine properties of the nanoparticles including its crystallinity, morphologies and sizes, and functional groups, respectively. The CV photodegradation process was carried out under different process conditions included initial CV concentration (10 mg/L – 25 mg/L), CV solution pH (5.39 – 8.98), and irradiation period (30 – 90 mins) to investigate the optimum operating conditions for the CV removal. The analysis using FESEM demonstrated that the nanoparticles exhibited irregularities and cylindrical shapes, measuring 109 nm in size. Meanwhile, the XRD analysis indicated that the iron oxide nanoparticles possessed a tetragonal crystal structure. The presence of Fe-O stretching vibrations at 486 cm-1 was confirmed by the FTIR spectrum. In terms of CV photodegradation studies, the optimum operating conditions for CV removal using iron oxide nanoparticles were determined to be at initial CV concentration of 10 mg/L, solution pH of 8.98, and an irradiation period of 90 mins, with a percentage removal of 96.21 %.
References
M.T. Yagub, T.K. Sen, S. Afroze, H.M. Ang, Dye and its removal from aqueous solution by adsorption: A review, in: Advances in Colloid and Interface Science, Elsevier, 2014: pp. 172–184. https://doi.org/10.1016/j.cis.2014.04.002.
O. Turgay, G. Ersoz, S. Atalay, J. Forss, U. Welander, The treatment of azo dyes found in textile industry wastewater by anaerobic biological method and chemical oxidation, Separation and Purification Technology 79 (2011) 26–33. https://doi.org/10.1016/j.seppur.2011.03.007.
S. Singh, S.L. Lo, V.C. Srivastava, A.D. Hiwarkar, Comparative study of electrochemical oxidation for dye degradation: Parametric optimization and mechanism identification, Journal of Environmental Chemical Engineering 4 (2016) 2911–2921. https://doi.org/10.1016/j.jece.2016.05.036.
D. Bhatia, N.R. Sharma, J. Singh, R.S. Kanwar, Biological methods for textile dye removal from wastewater: A review, Critical Reviews in Environmental Science and Technology 47 (2017) 1836–1876. https://doi.org/10.1080/10643389.2017.1393263.
A. Ghaffar, L. Zhang, X. Zhu, B. Chen, Porous PVdF/GO nanofibrous membranes for selective separation and recycling of charged organic dyes from water, Environmental Science and Technology 52 (2018) 4265–4274. https://doi.org/10.1021/acs.est.7b06081.
R. Cristovao, C. Botelho, R. Martins, R. Boaventura, Pollution prevention and wastewater treatment in fish canning industries of Northern Portugal, International Proceedings of Chemical, Biological and Environmental Engineering 32 (2012) 12–16. https://doi.org/10.7763/IPCBEE.
O. Sacco, V. Vaiano, C. Han, D. Sannino, D.D. Dionysiou, Photocatalytic removal of atrazine using N-doped TiO2 supported on phosphors, Applied Catalysis B: Environmental 164 (2015) 462–474. https://doi.org/10.1016/j.apcatb.2014.09.062.
O. Sacco, V. Vaiano, L. Rizzo, D. Sannino, Photocatalytic activity of a visible light active structured photocatalyst developed for municipal wastewater treatment Journal of Cleaner Production. 175 (2018) 38–49. https://doi.org/10.1016/j.jclepro.2017.11.088.
S.Y. Lee, D. Kang, S. Jeong, H.T. Do, J.H. Kim, Photocatalytic degradation of Rhodamine B dye by TiO2 and gold nanoparticles supported on a floating porous polydimethylsiloxane sponge under ultraviolet and visible light irradiation, ACS Omega 5 (2020) 4233-4241. https://doi.org/10.1021/acsomega.9b04127.
M.S.H. Bhuiyan, M.Y. Miah, S.C. Paul, T.D. Aka, O. Saha, M.M. Rahaman, J.I. Sharif, O. Habiba, M. Ashaduzzaman, Green synthesis of iron oxide nanoparticle using Carica papaya leaf extract: application for photocatalytic degradation of remazol yellow RR dye and antibacterial activity Heliyon. 6 (2020) e04603. https://doi.org/10.1016/j.heliyon.2020.e04603.
V.K. Nathan, P. Ammini, J. Vijayan, Photocatalytic degradation of synthetic dyes using iron (III) oxide nanoparticles (Fe2O3-Nps) synthesised using Rhizophora mucronata Lam, IET Biotechnology 13 (2019) 120–123. https://doi.org/10.1049/iet-nbt.2018.5230.
Z.A. Piranshahi, M. Behbahani, F. Zeraatpisheh, Synthesis, characterization and photocatalytic application of TiO2/magnetic graphene for efficient photodegradation of crystal violet, Applied Organometallic Chemistry 32 (2017) e3985. https://doi.org/10.1002/aoc.3985.
K. Mishra, N. Basavegowda, Y.R. Lee, Biosynthesis of Fe, Pd, and Fe-Pd bimetallic nanoparticles and their application as recyclable catalysts for [3 + 2] cycloaddition reaction: A comparative approach, Catalysis Science and Technology 5 (2015) 2612–2621. https://doi.org/10.1039/c5cy00099h.
V. Gopinath, S. Priyadarshini, N. Meera Priyadharsshini, K. Pandian, P. Velusamy, Biogenic synthesis of antibacterial silver chloride nanoparticles using leaf extracts of Cissus quadrangularis Linn, Materials Letters 91 (2013) 224–227. https://doi.org/10.1016/j.matlet.2012.09.102.
D. Mishra, R. Arora, S. Lahiri, S.S. Amritphale, N. Chandra, Synthesis and characterization of iron oxide nanoparticles by solvothermal method, Protection of Metals and Physical Chemistry of Surfaces 50 (2014) 628–631. https://doi.org/10.1134/S2070205114050128.
X. Zheng, Y. Jiao, F. Chai, F. Qu, A. Umar, X. Wu, Template-free growth of well-crystalline a-Fe2O3 nanopeanuts with enhanced visible-light driven photocatalytic properties, Journal of Colloid and Interface Science 457 (2015) 345–352. https://doi.org/10.1016/j.jcis.2015.07.023.
M. Jamzad, M. Kamari Bidkorpeh, Green synthesis of iron oxide nanoparticles by the aqueous extract of Laurus nobilis L. leaves and evaluation of the antimicrobial activity, Journal of Nanostructure in Chemistry 10 (2020) 193–201. https://doi.org/10.1007/s40097-020-00341-1.
H. Veisi, L. Mohammadi, S. Hemmati, T. Tamoradi, P. Mohammadi, In situ immobilized silver nanoparticles on Rubia tinctorum extract-coated ultrasmall iron oxide nanoparticles: An efficient nanocatalyst with magnetic recyclability for synthesis of propargylamines by A3 coupling reaction, ACS Omega 4 (2019) 13991–14003. https://doi.org/10.1021/acsomega.9b01720.
S.M. Shalaby, F.F. Madkour, H.Y. El-Kassas, A.A. Mohamed, A.M. Elgarahy, Green synthesis of recyclable iron oxide nanoparticles using Spirulina platensis microalgae for adsorptive removal of cationic and anionic dyes, Environmental Science and Pollution Research 28 (2021) 65549–65572. https://doi.org/10.1007/s11356-021-15544-4.
M.V. Arularasu, J. Devakumar, T.V. Rajendran, An innovative approach for green synthesis of iron oxide nanoparticles: Characterization and its photocatalytic activity, Polyhedron 156 (2018) 279–290. https://doi.org/10.1016/j.poly.2018.09.036.
Z. Talib, A. Al-Kadum, Optimization of degradation crystal violet utilizing photocatalyst ZnO/UV Light, Journal University of Kerbala 16 (2018) 10-19.
A. Jouali, A. Salhi, A. Aguedach, A. Aarfane, H. Ghazzaf, E.K. Lhadi, M. el Krati, S. Tahiri, Photo-catalytic degradation of methylene blue and reactive blue 21 dyes in dynamic mode using TiO2 particles immobilized on cellulosic fibers, Journal of Photochemistry and Photobiology A: Chemistry 383 (2019). https://doi.org/10.1016/j.jphotochem.2019.112013.
K.M. Reza, A. Kurny, F. Gulshan, Parameters affecting the photocatalytic degradation of dyes using TiO2: A review, Applied Water Science 7 (2017) 1569–1578. https://doi.org/10.1007/s13201-015-0367-y.
S. Wardhani, D. Purwonugroho, C.W. Fitri, Y.P. Prananto, Effect of pH and irradiation time on TiO2-chitosan activity for phenol photo-degradation, AIP Conference Proceedings 2021 (2018) 1–7. https://doi.org/10.1063/1.5062759.
A.M. Abdullah, N.J. Al-Thani, K. Tawbi, H. Al-Kandari, Carbon/nitrogen-doped TiO2: New synthesis route, characterization and application for phenol degradation, Arabian Journal of Chemistry 9 (2016) 229–237. https://doi.org/10.1016/j.arabjc.2015.04.027.
A. Kumar, G. Pandey, The photocatalytic degradation of methyl green in presence of visible light with photoactive Ni 0.10: La 0.05: TiO2 nanocomposites, IOSR Journal of Applied Chemistry 10 (2017), 31–44. https://doi.org/10.9790/5736-1009013144.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2023 Progress in Energy and Environment

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.











