Synthesis of Bi-Component ZrO2/Ag Nanotube for Heavy Metal Removal
DOI:
https://doi.org/10.37934/progee.18.1.2333Keywords:
ZrO2/Ag , Nanotubes, Anodization, Photoreduction, AdsorptionAbstract
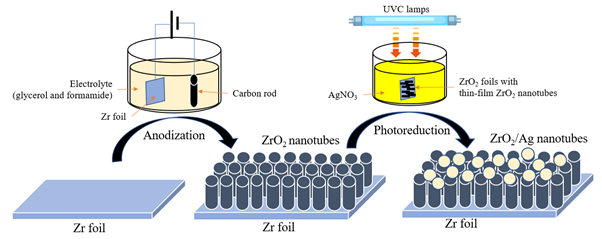
This study was conducted to synthesize bi-component ZrO2/Ag nanotubes through anodization and photoreduction methods. The synthesized nanotubes were characterized and adsorption tests were carried out to evaluate its performance in removing heavy metal, lead (II). ZrO2 nanotubes were synthesized by anodizing zirconium foil in an electrolyte composed of glycerol, ammonium fluoride, formamide, and distilled water. The effect of anodizing time and the annealing process on the morphology of synthesized nanotubes were studied. Bi-component ZrO2/Ag nanotubes were prepared through photochemical reduction which silver precursor solution undergoes Ultraviolet (UV) irradiation in the presence of the active reducing agent. Larger pore diameter and longer length of synthesized nanotubes were formed at the longer anodizing time and the walls of nanotubes were smoother without annealing. The effect of the initial heavy metal concentration and contact time on the adsorption efficiency of synthesized nanotubes was evaluated using lead (II) as the heavy metal ions. Overall, the percentage removal of lead (II) increased with longer adsorption time and higher initial concentration of the lead (II) ions.
References
K. Gupta, P. Joshi, R. Gusain, O.P. Khatri, Recent advances in adsorptive removal of heavy metal and metalloid ions by metal oxide-based nanomaterial, Coordination Chemistry Reviews 445 (2021) 214100. https://doi.org/10.1016/j.ccr.2021.214100.
A.M. Awad, R. Jalab, A. Benamor, M.S. Nasser, M.M. Ba-Abbad, M. El-Naas, A.W. Mohammad, Adsorption of organic pollutants by nanomaterial-based adsorbents: An overview, Journal of Molecular Liquids 301 (2020) 112335. https://doi.org/10.1016/j.molliq.2019.112335.
J. Liu, Z. Zhao, C. Xu, J. Liu, Structure, synthesis, and catalytic properties of nanosize cerium-zirconium-based solid solutions in environmental catalysis, Chinese Journal of Catalysis 40(10) (2019) 1438–1487. http://dx.doi.org/10.1016/S1872-2067(19)63400-5.
J. Yang, B. Hou, J. Wang, B. Tian, J. Bi, N. Wang, X. Lin, X. Huang, Nanomaterials for the removal of heavy metals from wastewaterm Nanomaterials 9(3) (2019). https://doi.org/10.3390/nano9030424.
N.X. Dinh, D.T. Chi, N.T. Lan, H. Lan, H.V. Tuan, N.V. Quy, V.N. Phan, T.Q. Huy, A. Le, Water-dispersible silver nanoparticles-decorated carbon nanomaterials: synthesis and enhanced antibacterial activity, Applied Physics A: Materials Science and Processing 119(1) (2015) 85–95. https://doi.org/10.1007/s00339-014-8962-6.
E. Sumesh, M.S. Bootharaju, Anshup, T. Pradeep, A practical silver nanoparticle-based adsorbent for the removal of Hg2+ from water, Journal of Hazardous Materials, 189(1–2) (2011) 450–457. https://doi.org/10.1016/j.jhazmat.2011.02.061.
T. Wen, H. Tan, S. Chen, P. He, S. Yang, C. Deng, and S. Liu, Growth behavior of tantalum oxide nanotubes during constant current anodization, Electrochemistry Communications 128 (2021) 107073. https://doi.org/10.1016/j.elecom.2021.107073.
Y. Ni, J. Zhang, T. Gong, M. Sun, Z. Zhao, X. Li, H. Yu, X. Zhu, Quantitative analysis of the volume expansion of nanotubes during constant voltage anodization, Surfaces and Interfaces, 26 (2021) 101419. https://doi.org/10.1016/j.surfin.2021.101419.
M.S.A. Berahim, M.F. Majnis, M.A.A. Taib, Formation of titanium dioxide nanoparticles by anodization of valve metals, Progress in Energy and Environment 7 (2019) 11–19. https://www.akademiabaru.com/submit/index.php/progee/article/view/1050.
M. D. L. Balela, C. Mancera, B. P. Reyes, M.C. Reyes, Anodization of zirconia nanotubes for lead (II) adsorption, Materials Science Forum, 939 (2018) 113–119. https://doi.org/10.4028/www.scientific.net/MSF.939.113.
M. Shkir, M. T. Khan, I. M. Ashraf, S. AlFaify, A.M. El-Toni, A. Aldalbahi, H. Ghaithan, A. Khan, Rapid microwave-assisted synthesis of Ag-doped PbS nanoparticles for optoelectronic applications, Ceramics International 45(17) (2019) 21975–21985. https://doi.org/10.1016/j.ceramint.2019.07.212.
N. Thakur, Anu, K. Kumar, Effect of (Ag, Co) co-doping on the structural and antibacterial efficiency of CuO nanoparticles: A rapid microwave assisted method, Journal of Environmental Chemical Engineering 8(4) (2020) 1–9. https://doi.org/10.1016/j.jece.2020.104011.
U. Kamran, S.J. Park, Microwave-assisted acid functionalized carbon nanofibers decorated with Mn doped TNTs nanocomposites: Efficient contenders for lithium adsorption and recovery from aqueous media, Journal of Industrial and Engineering Chemistry, 92 (2020) 263–277. https://doi.org/10.1016/j.jiec.2020.09.014.
M.Z. Toe, A.T. Le, S.S. Han, K.A.B. Yaacob, S.Y. Pung, Silver nanoparticles coupled ZnO nanorods array prepared using photo-reduction method for localized surface plasmonic effect study, Journal of Crystal Growth 547 (2020) 125806. https://doi.org/10.1016/j.jcrysgro.2020.125806.
F. Lu, J. Wang, Z. Chang, J. Zeng, Uniform deposition of Ag nanoparticles on ZnO nanorod arrays grown on polyimide/Ag nanofibers by electrospinning, hydrothermal, and photoreduction processes, Materials and Design 181 (2019) 108069. https://doi.org/10.1016/j.matdes.2019.108069.
M. Jodeyri, M. Haghighi, M. Shabani, Plasmon-assisted demolition of antibiotic using sono-photoreduction decoration of Ag on 2D C3N4 nanophotocatalyst enhanced with acid-treated clinoptilolite, Ultrasonics Sonochemistry 54 (2019) 220–232. https://doi.org/10.1016/j.ultsonch.2019.01.035.
R. Gupta, N.K.R. Eswar, J.M. Modak, G. Madras, Ag and CuO impregnated on Fe doped ZnO for bacterial inactivation under visible light, Catalysis Today 300 (2018) 71–80. http://dx.doi.org/10.1016/j.cattod.2017.05.032.
L. Zhou, J. Yang, X. Wang, G. Song, F. Lu, L. You, J. Li, Ag nanoparticles decorated Ag@ZrO2 composite nanospheres as highly active SERS substrates for quantitative detection of hexavalent chromium in waste water, Journal of Molecular Liquids 319 (2020) 114158. https://doi.org/10.1016/j.molliq.2020.114158.
M. Maham, M. Nasrollahzadeh, S. Mohammad Sajadi, Facile synthesis of Ag/ZrO2 nanocomposite as a recyclable catalyst for the treatment of environmental pollutants, Composites Part B: Engineering 185 (2020) 107783. https://doi.org/10.1016/j.compositesb.2020.107783.
P. Rajapandiyan, J. Yang, Photochemical method for decoration of silver nanoparticles on filter paper substrate for SERS application, Journal of Raman Spectroscopy 45(7) (2014) 574–580. https://doi.org/10.1002/jrs.4502.
S.Z. Qiao, J. Liu, G.Q. Max Lu, Synthetic Chemistry of Nanomaterials. Modern Inorganic Synthetic Chemistry, Second Edition, Elsevier B.V., 2017. http://dx.doi.org/10.1016/B978-0-444-63591-4.00021-5
V.R. Dandu Kamakshi Gari, M. Kim, Removal of Pb(II) using silver nanoparticles deposited graphene oxide: equilibrium and kinetic studies, Monatshefte fur Chemie 146(9) (2015) 1445–1453. https://doi.org/10.1007/s00706-015-1429-4.
P. Van Viet, B. Thang Phan, D. Mott, S. Maenosono, T. Tan Sang, C. Minh Thi, L. Van Hieu, Silver nanoparticle loaded TiO2 nanotubes with high photocatalytic and antibacterial activity synthesized by photoreduction method, Journal of Photochemistry and Photobiology A: Chemistry 352 (2018) 106–112. https://doi.org/10.1016/j.jphotochem.2017.10.051.
M. Wang, L. Jia, and S. Deng, Influence of anode area and electrode gap on the morphology of TiO2 nanotubes arrays 534042 (2013). https://doi.org/10.1155/2013/534042.
N. Chaouch, M.R. Ouahrani, S.E. Laouini, Adsorption of Lead (II) from aqueous solutions onto activated carbon prepared from algerian dates stones of phoenix dactylifera L (Ghars variety) by H3PO4 activation, Oriental Journal of Chemistry 30(3) (2014) 1317-1322. http://dx.doi.org/10.13005/ojc/300349
K. Yasuda, P. Schmuki, Formation of self-organized zirconium titanate nanotube layers by alloy anodization, Advanced Materials 19(13) (2007) 1757–1760. https://doi.org/10.1002/adma.200601912.
L. Guo, J. Zhao, X. Wang, X. Xu, H. Liu, Y. Li, Structure and bioactivity of zirconia nanotube arrays fabricated by anodization, International Journal of Applied Ceramic Technology 6(5) (2009) 636–641. https://doi.org/10.1111/j.1744-7402.2008.02305.x.
N. Bashirom, K.A. Razak, C.K. Yew, Z. Lockman, Effect of Fluoride or Chloride ions on the morphology of ZrO2 thin film grown in ethylene glycol electrolyte by anodization, Procedia Chemistry 19 (2016) 611–618. http://dx.doi.org/10.1016/j.proche.2016.03.060.
X. Pan, M.Q. Yang, X. Fu, N. Zhang, Y.J. Xu, Defective TiO2 with oxygen vacancies: Synthesis, properties and photocatalytic applications, Nanoscale 5(9) (2013) 3601–3614. https://doi.org/10.1039/C3NR00476G.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2022 Progress in Energy and Environment

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.











