The Effects of Magnetization Process on Methylene Blue Removal using Magnetically Modified Orange Peel
DOI:
https://doi.org/10.37934/progee.17.1.116Keywords:
Methylene blue, Magnetized orange peel, Fractional factorial design, Regeneration, FTIRAbstract
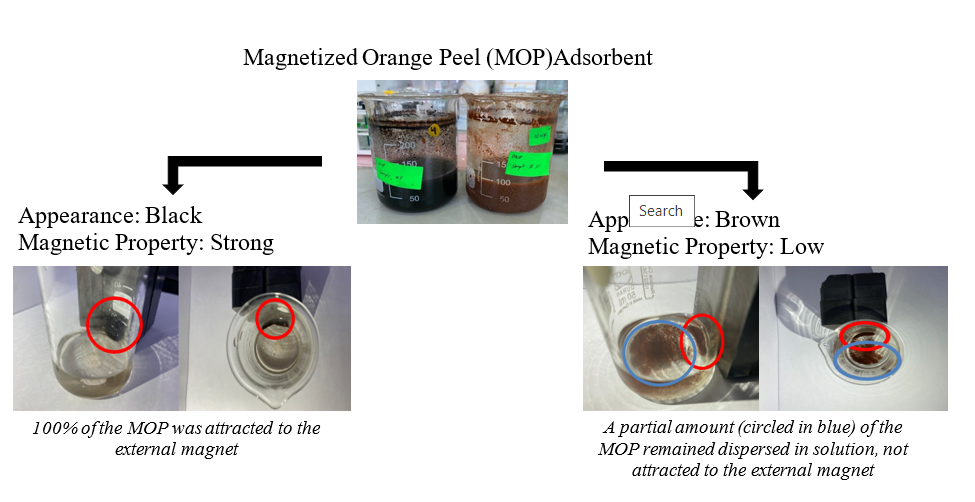
Whilst adsorption process is the preferred method of purifying wastewater due to its benefits, problems with the recovery of spent adsorbents are still prevalent in wastewater treatment technology. The use of magnetized biomass-based adsorbents (biosorbents) to ease the regeneration process would be a novel approach to overcome this obstacle. The magnetization of orange peel adsorbent involves a series of preparation stages. In this context, there are several parameters that may affect the magnetization of orange peel (OP) such as the ratio between FeCl3•6H2O and FeCl2•4H2O, mass of untreated orange peel (UOP), volume of NH3 solution, magnetization temperature and magnetization period. In this study, Fractional Factorial Design (FFD) was adopted to identify the significant parameters affecting two different responses namely the success of magnetization process and methylene blue (MB) dye removal. Based on the ANoVA results, the significant parameters affecting the success of the magnetization process were magnetization temperature, interaction between ratio of FeCl2:FeCl3 and volume of ammonia, and mass of OP with duration of mixing. Whereas the significant parameters affecting the MB dye removal were all five of the individual parameters, along with the interaction of amount of OP with the other four parameters, interaction between volume of ammonia with duration of mixing and with ratio of FeCl2:FeCl3, interaction between duration of mixing with temperature and ratio of FeCl2:FeCl3, and interaction between temperature and ratio of FeCl2:FeCl3. The highest recorded MB removal was 89.18%, while the lowest recorded MB removal was 38.76%. The regeneration study also showed that magnetized orange peel could be regenerated at least six times without having a significant reduction in adsorption capacity. The major functional groups of magnetized orange peel before adsorption, after adsorption and after regeneration were all similar, indicating that the spent adsorbent could be regenerated.
References
USGS, How much water is there on, in, and above the Earth? (2016) Retrieved from http://water.usgs.gov/edu/earthhowmuch.html.
Bureau of Reclamation, Water facts–Worldwide water supply (2020) Retrieved from https://www.usbr.gov/mp/arwec/water-facts-ww-water-sup.html.
T. Khokhar, Chart: Globally, 70% of freshwater is used for agriculture (2017) Retrieved from
https://blogs.worldbank.org/opendata/chart-globally-70-freshwater-used-agriculture.
I. Anastopoulos, G. Z. Kyzas, Agricultural peels for dye adsorption: A review of recent literature. Journal of Molecular Liquids, 200 (2014) 381–389. https://doi.org/10.1016/j.molliq.2014.11.006.
M. Hassanpour, H. Safardoust-Hojaghan, M. Salavati-Niasary, 2017. Degradation of methylene blue and Rhodamine B as water pollutants via green synthesized Co3O4/ZnO nanocomposite. Journal of Molecular Liquids, 229 (2017) 293–299. https://doi.org/10.1016/j.molliq.2016.12.090.
G. Crini, E. Lichtfouse, Advantages and disadvantages of techniques used for wastewater treatment. Environmental Chemistry Letters, 17 (2019) 145–155. https://doi.org/10.1007/s10311-018-0785-9.
A. Abbas, S. Murtaza, M. Munir, T. Zahid, N. Abbas, A. Mushtaq, Removal of congo red from aqueous solutions with Raphanus sativus peels and activated carbon: A comparative study American-Eurasian. Journal of Agriculture & Environmental Science, 10(5) (2011) 802–809. https://www.semanticscholar.org/paper/Removal-of-Congo-Red-from-aqueous-solutions-with-a-Abbas-Murtaza/477a5ab65acedc56665eaac2b41bb069f6808963.
I. Anastopoulos, I. Pashalidis, A. Hosseini-Bandegharaei, D. A. Giannakoudakis, A. Robalds, M. Usman, L. B. Escudero, Y. Zhou, J. C. Colmenares, A. Nunez-Delgado, E. C. Lima, Agricultural biomass/waste as adsorbents for toxic metal decontamination of aqueous solutions. Journal of Molecular Liquids, 295 (2019) 111684. https://doi.org/10.1016/j.molliq.2019.111684.
S. Daneshfozoun, M. A. Abdullah, B. Abdullah, Preparation and characterization of magnetic biosorbent based on oil palm empty fruit bunch fibers, cellulose and ceiba pentandra for heavy metal ions removal. Industrial Crops & Products, 105 (2017) 93–103. https://doi.org/10.1016/j.indcrop.2017.05.011.
M. O. Omorogie, J. O. Babalola, E. I. Unuabonah, Regeneration strategies for spent solid matrices used in adsorption of organic pollutants from surface water: A critical review. Desalination and Water Treatment, 57(2) (2014) 518–544. https://doi.org/10.1080/19443994.2014.967726.
Y. Bulut, H. Aydin, A kinetics and thermodynamics study of methylene blue adsorption on wheat shells. Desalination, 194(1–3) (2006) 259–267. https://doi.org/10.1016/j.desal.2005.10.032.
H. Karaer, I. Kaya, Synthesis, characterization of magnetic chitosan/active charcoal composite and using at the adsorption of methylene blue and reactive blue4. Microporous and Mesoporous Materials, 232 (2016) 26–38. https://doi.org/10.1016/j.micromeso.2016.06.006.
T. S. Jong, C. Y. Yoo, P. L. Kiew, Feasibility study of methylene blue adsorption using magnetized papaya seeds. Progress in Energy and Environment, 14 (2020) 1–12. https://www.akademiabaru.com/submit/index.php/progee/article/view/909/2731.
M. H. Munawer, H. L. Chee, P. L. Kiew, Magnetized orange peel: A realistic approach for methylene blue removal. Materials Today: Proceedings (2021). https://doi.org/10.1016/j.matpr.2021.02.796.
H. L. Chee, Comparative study of methylene blue removal using magnetic modified, chemically treated and untreated orange peel: Adsorption and kinetic studies. [Unpublished bachelor’s thesis]. UCSI University (2017).
P. L. Kiew, J. F. Toong, Screening of significant parameters affecting Zn (II) adsorption by chemically treated watermelon rind. Progress in Energy Environment, 6 (2018) 19–32. https://www.akademiabaru.com/submit/index.php/progee/article/view/1048/40.
N. C. Feng, X. Y. Guo, S. Liang, Enhanced Cu (II) adsorption by orange peel modified with sodium hydroxide-TNMSC. The Chinese Journal of Nonferrous Metals, 20(1) (2009) s146–s152. http://www.ysxbcn.com/down/upfile/soft/201076/29-p146.pdf.
K. Y. Foo, B. H. Hameed, Preparation, characterization and evaluation of adsorptive properties of orange peel based activated carbon via microwave induced K2CO3 activation. Bioresource Technology, 104 (2012) 679–686. https://doi.org/10.1016/j.biortech.2011.10.005.
M. Arami, N. Y. Limaee, N. M. Mahmoodi, N. S. Tabrizi, Removal of dyes from colored textile wastewater by orange peel adsorbent: Equilibrium and kinetic studies. Journal of Colloid and Interface Science, 288(2) (2005) 371–376. https://doi.org/10.1016/j.jcis.2005.03.020.
N. Feng, X. Guo, S. Liang, Adsorption study of copper (II) by chemically modified orange peel. Journal of Hazardous Materials, 164 (2–3) (2009) 1286–1292. https://doi.org/10.1016/j.jhazmat.2008.09.096.
A. E. Nemr, O. Abdelwahab, A. El-Sikaily, A. Khaled, Removal of direct blue-86 from aqueous solution by new activated carbon developed from orange peel. Journal of Hazardous Materials, 161(1) (2009) 102–110. https://doi.org/10.1016/j.jhazmat.2008.03.060.
X. Li, Y. Tang, X. Cao, D. Lu, F. Luo, W. Shao, Preparation and evaluation of orange peel cellulose adsorbents for effective removal of cadmium, zinc, cobalt and nickel. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 317(1–3) (2008) 512–521. https://doi.org/10.1016/j.colsurfa.2007.11.031.
D. Lu, Q. Cao, X. Li, X. Cao, F. Luo, W. Shao, Kinetics and equilibrium of Cu(II) adsorption onto chemically modified orange peel cellulose biosorbents. Hydrometallurgy, 95 (2009) 145–152. https://doi.org/10.1016/j.hydromet.2008.05.008.
V. K. Gupta, A. Nayak, Cadmium removal and recovery from aqueous solutions by novel adsorbents prepared from orange peel and Fe2O3 nanoparticles. Chemical Engineering Journal, 180 (2012) 81–90. https://doi.org/10.1016/j.cej.2011.11.006.
K. G. P. Nunes, L. W. Sfreddo, M. Rosset, L. A. Fèris, Efficiency evaluation of thermal, ultrasound and solvent techniques in activated carbon regeneration. Environmental Technology, (2020) 1–23. https://doi.org/10.1080/09593330.2020.1746839.
O. Hamdaoui, E. Naffrehoux, L. Tifouti, C. Petrier, Effects of ultrasound on adsorption–desorption of p-chlorophenol on granular activated carbon. Ultrasonics Sonochemistry, 10 (2003) 109–114. https://doi.org/10.1016/s1350-4177(02)00137-2.
J. L. Li, D. C. Li, S. L. Zhang, H. C. Cui, Z. Wang, Analysis of the factors affecting the magnetic characteristics of nano-Fe3O4 particles. Materials Science, 56 (2011) 803–810. https://doi.org/10.1007/s11434-010-4126-z.
A. L. Andrade, D. M. Souza, M. C. Pereira, J. D. Fabris, R. Z. Domingues, pH effect on the synthesis of magnetite nanoparticles by the chemical reduction-precipitation method. Química Nova, 33(3) (2010) 524–527. https://doi.org/10.1590/S0100-40422010000300006.
A. O. Dada, F. A. Adekola, O. S. Adeyemi, O. M. Bello, A. C. Oluwaseun, O. J. Awakan, F. A. Grace, Exploring the effect of operational factors and characterization imperative to the synthesis of silver nanoparticles. In Silver nanoparticles – Fabrication, characterization and applications, K. Maaz (Ed), Intechopen (2018). https://www.intechopen.com/chapters/61862.
P. Roonasi, A. Holmgren, A study on the mechanism of magnetite formation based on iron isotope fractionation. (2009) Retrieved from https://www.diva-portal.org/smash/get/diva2:1010902/FULLTEXT01.pdf.
Y. Chen, Q. Chen, H. Mao, Y. Lin, J. Li, Preparation of magnetic nanoparticles via a chemically induced transition: Presence/Absence of magnetic transition on the treatment solution used. Journal of Chemistry, 9 (2016). https://doi.org/10.1155/2016/7604748.
S. P. Schwaminger, C. Syhr, S. Berensmeier, Controlled synthesis of magnetic iron oxide nanoparticles: Magnetite or Maghemite? Crystals, 10(3) (2020) 214. https://doi.org/10.3390/cryst10030214.
H. Panda, N. Tiadi, M. Mohanty, C. R. Mohanty, Studies on adsorption behavior of an industrial waste for removal of chromium from aqueous solution. South African Journal of Chemical Engineering, 23 (2017) 132–138. https://doi.org/10.1016/j.sajce.2017.05.002.
B. Kalska-Szotsko, U. Wykowska, K. Piekut, D. Satula, Stability of Fe3O4 nanoparticles in various model solutions. Colloids and Surfaces A: Physicochemical and Engineering Aspects, 450 (2014) 15–24. https://doi.org/10.1016/j.colsurfa.2014.03.002.
S. E. Favela-Camacho, J. F. Pérez-Robles, P. E. Garciá-Casillas, A. Godinez-Garcia, Stability of magnetite nanoparticles with different coatings in a simulated blood plasma. Journal of Nanoparticle Research, 18 (2016) 176. https://doi.org/10.1007/s11051-016-3482-2.
R. Kheshtzar, A. Berenjian, S. M. Taghizadeh, Y. Ghasemi, A. G. Asad, A. Ebrahiminezhad, Optimization of reaction parameters for the green synthesis of zero valent iron nanoparticles using pine tree needles. Green Processing and Synthesis, 8(1) (2019) 846–855. https://doi.org/10.1515/gps-2019-0055.
N. Othman, A. S. Che-Azhar, A. Suhaimi, Zinc removal using honey dew rind. Applied Mechanics and Materials, 680 (2014) 150–153. https://doi.org/10.4028/www.scientific.net/AMM.680.150.
E. A. Setiadi, N. S. Asri, Y. A. Wijayanti, A. P. Tetuko, P. Sebayang, P., The effect of ammonia solution concentration on the synthesis process of MgFe2O4 based on natural iron sand as adsorbent of Pb ions. AIP Conference Proceedings, 2256 (2020) 030011. https://doi.org/10.1063/5.0015497.
D. Maity, J. Ding, J. M. Xue, Synthesis of magnetite nanoparticles by thermal decomposition: Time, temperature, surfactant and solvent effects. World Scientific Publishing Company, 1(3) (2008) 189–193. https://doi.org/10.1142/S1793604708000381.
S. Yean, L. Cong, C. T. Yavuz, J. T. Mayo, W. W. Yu, A. T. Kan, V. T. Colvin, M. B. Tomson, Effect of magnetite particle size on adsorption and desorption of arsenite and arsenate. Journal of Materials Research, 20(12) (2005) 3255–3264. https://doi.org/10.1557/jmr.2005.0403.
N. Mizutani, T. Iwasaki, S. Watano, T. Yanagida, H. Tanaka, T. Kawai, Effect of ferrous/ferric ions molar ratio on reaction mechanism for hydrothermal synthesis of magnetite nanoparticles. Bulletin of Materials Science, 31(5) (2008) 713–717. https://doi.org/10.1007/s12034-008-0112-3.
L. Hou, Q. Liang, F. Wang, Mechanisms that control the adsorption-desorption behavior of phosphate on magnetite nanoparticles: The role of particle size and surface chemistry characteristics. Royal Society of Chemistry, 10 (2020) 2378–2388. https://doi.org/10.1039/C9RA08517C.
M. Hassan, R. Naidu, J. Du, Y. Liu, F. Qi, Critical review of magnetic biosorbents: their preparation, application, and regeneration for wastewater treatment. Science of the Total Environment, 702 (2019) 134893. https://doi.org/10.1016/j.scitotenv.2019.134893.
P. Verma, S. K. Samanta, A direct method to determine the adsorbed dyes on adsorbent via processing of diffuse reflectance spectroscopy data. Materials Research Express, 6 (2019) 015505. https://doi.org/10.1088/2053-1591/aae3f2.
L. Liu, Z. Gao, X. Su, X. Chen, L. Jiang, J. Yao, Adsorption removal of dyes from single and binary solutions using a cellulose-based bioadsorbents. ACS Sustainable Chemistry and Engineering, 3(3) (2015) 432–442. https://doi.org/10.1021/sc500848m.
W. Huang, Y. Hu, Y. Li, Y. Zhou, D. Niu, Z. Lei, Z. Zhang, Citric acid-crosslinked B-cyclodextrin for simultaneous removal of bisphenol A, methylene blue and copper: The roles of cavity and surface functional groups. Journal of the Taiwan Institute of Chemical Engineers, 82 (2018) 189–197. https://doi.org/10.1016/j.jtice.2017.11.021.
T. Ainane, K. Fatima, M. Talbi, M. Elkouali, A novel bio-adsorbent of mint waste for dyes remediation in aqueous environments: Study and modeling of isotherms for removal of Methylene Blue. Oriental Journal of Chemistry, 30(3) (2014) 1183–1189. http://dx.doi.org/10.13005/ojc/300332.
A. Mohd Nor, T. Hadibarata, Z. Yusop, Z. Mat Lazim, Removal of brilliant green and procionred dyes from aqueous solutionby adsorption using selected agricultural wastes. Jurnal Teknologi, 74(11) (2015) 117–122. https://doi.org/10.11113/jt.v74.4880.
M. Boumediene, H. Benaissa, B. George, S. Molina, A. Merlin, Characterization of two cellulosic waste materials (orange and almond peels) and their use for the removal of methylene blue from aqueous solutions. Maderas. Ciencia y tecnología, 17(1) (2015) 69–84. http://dx.doi.org/10.4067/S0718-221X2015005000008.
S. T. Yang, S. Chen, Y. Chang, A. Cao, Y. Liu, F. Wang, Removal of methylene blue from aqueous solution by graphene oxide. Journal of Colloid and Interface Science, (2011) 24–29. https://doi.org/10.1016/j.jcis.2011.02.064.
Chemistry LibreTexts, Infrared Spectroscopy Absorption Table (2020). Retrieved from https://chem.libretexts.org/@go/page/22645.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2021 Progress in Energy and Environment

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.











