Preliminary Investigation on the Disruption of Microalgae Cell Wall using Vortex Induced Vibration (VIV)
Keywords:
Scenedesmus, Vortex Induced Vibration (VIV), TurbidityAbstract
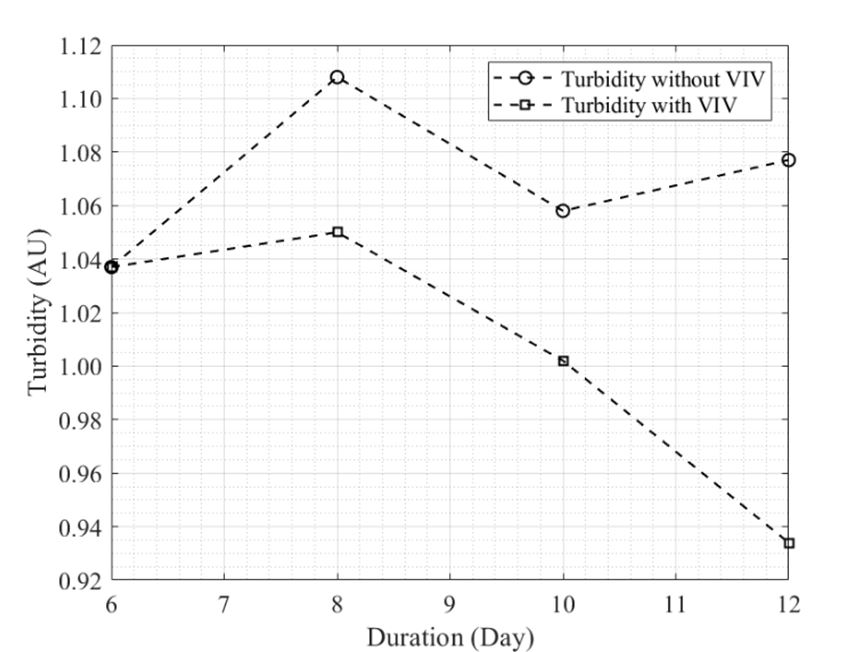
Scenedesmus sp. is industrially known for its high lipids content that can be used in biofuels production. Most of the conventional mechanical methods to disrupt the microalgae cell wall use high frequency approaches. The conventional high frequency methods have few disadvantages which are high energy consumption, high cost and application of solvents which are the cause of environmental pollution. In this paper, a low frequency method called vortex induced vibration (VIV) is proposed to replace the conventional mechanical methods to disrupt microalgae cell wall. An experimental rig has been designed and fabricated for this experiment. Based on the experiment, the result shows that VIV method has the possibility to break microalgae cell wall since the turbidity decrease throughout the days.
References
Y.M. Kong, J.H. Lee, K.M. Lee, W.Y. Tey, Techniques of improving microalgae in biomass clean energy: A short review, Progress in Energy and Environment. 10 (2019) 6–20.
S.P. Choi, M.T. Nguyen, S.J. Sim, Enzymatic pretreatment of Chlamydomonas reinhardtii biomass for ethanol production, Bioresource Technology. 101 (2010) 5330–5336. https://doi.org/10.1016/j.biortech.2010.02.026.
C.-Y. Chen, K.-L. Yeh, R. Aisyah, D.-J. Lee, J.-S. Chang, Cultivation, photobioreactor design and harvesting of microalgae for biodiesel production: A critical review, Bioresource Technology. 102 (2011) 71–81. https://doi.org/10.1016/j.biortech.2010.06.159.
M.A. Borowitzka, High-value products from microalgae—their development and commercialisation, Journal of Applied Phycology. 25 (2013) 743–756. https://doi.org/10.1007/s10811-013-9983-9.
E. Günerken, E. D’Hondt, M.H.M. Eppink, L. Garcia-Gonzalez, K. Elst, R.H. Wijffels, Cell disruption for microalgae biorefineries, Biotechnology Advances. 33 (2015) 243–260. https://doi.org/10.1016/j.biotechadv.2015.01.008.
M.M. Mendes-Pinto, M.F.J. Raposo, J. Bowen, A.J. Young, R. Morais, Evaluation of different cell disruption processes on encysted cells of Haematococcus pluvialis: effects on astaxanthin recovery and implications for bio-availability, Journal of Applied Phycology. 13 (2001) 19–24. https://doi.org/10.1023/A:1008183429747.
C. Dixon, L.R. Wilken, Green microalgae biomolecule separations and recovery, Bioresources and Bioprocessing. 5 (2018) Article Number 14. https://doi.org/10.1186/s40643-018-0199-3.
M.D.A. Zbinden, B.S.M. Sturm, R.D. Nord, W.J. Carey, D. Moore, H. Shinogle, S.M. Stagg-Williams, Pulsed electric field (PEF) as an intensification pretreatment for greener solvent lipid extraction from microalgae, Biotechnology and Bioengineering. 110 (2013) 1605–1615. https://doi.org/10.1002/bit.24829.
F.J. Barba, N. Grimi, E. Vorobiev, New Approaches for the Use of Non-conventional Cell Disruption Technologies to Extract Potential Food Additives and Nutraceuticals from Microalgae, Food Engineering Reviews. 7 (2015) 45–62. https://doi.org/10.1007/s12393-014-9095-6.
A. A, S. Kumari, P. Turkar, S. Subramanian, An insight on algal cell distruption for biodiesel production, Asian Journal of Pharmaceutical and Clinical Research. 11 (2018). https://doi.org/10.22159/ajpcr.2018.v11i2.22481.
S.T.L. Harrison, Bacterial cell disruption: A key unit operation in the recovery of intracellular products, Biotechnology Advances. 9 (1991) 217–240. https://doi.org/10.1016/0734-9750(91)90005-G.
A.K. Lee, D.M. Lewis, P.J. Ashman, Disruption of microalgal cells for the extraction of lipids for biofuels: Processes and specific energy requirements, Biomass and Bioenergy. 46 (2012) 89–101. https://doi.org/10.1016/j.biombioe.2012.06.034.
M. al Hattab, G. Abdel, H. Amal, Microalgae Harvesting Methods for Industrial Production of Biodiesel: Critical Review and Comparative Analysis, Journal of Fundamentals of Renewable Energy and Applications. 05 (2015). https://doi.org/10.4172/2090-4541.1000154.
P. Biller, C. Friedman, A.B. Ross, Hydrothermal microwave processing of microalgae as a pre-treatment and extraction technique for bio-fuels and bio-products, Bioresource Technology. 136 (2013) 188–195. https://doi.org/10.1016/j.biortech.2013.02.088.
V. Pasquet, J.-R. Chérouvrier, F. Farhat, V. Thiéry, J.-M. Piot, J.-B. Bérard, R. Kaas, B. Serive, T. Patrice, J.-P. Cadoret, L. Picot, Study on the microalgal pigments extraction process: Performance of microwave assisted extraction, Process Biochemistry. 46 (2011) 59–67. https://doi.org/10.1016/j.procbio.2010.07.009.
M. Costalonga, P. Brunet, H. Peerhossaini, Low frequency vibration induced streaming in a Hele-Shaw cell, Physics of Fluids. 27 (2015). https://doi.org/10.1063/1.4905031.
P.E. Wiley, Microalgae cultivation using offshore membrane enclosures for growing algae (OMEGA), 2013. https://escholarship.org/uc/item/0586c8p5 (accessed January 17, 2021).
N. Bakuei, G. Amini, G.D. Najafpour, M. Jahanshahi, M. Mohammadi, Optimal cultivation of Scenedesmus sp. microalgae in a bubble column photobioreactor, Indian Journal of Chemical Technology. 22 (2015) 20–25.
P.Y. Yap, A. Jain, D. Trau, Determination of biomass in spirulina cultures by photopette, 2018. https://tipbiosystems.com/wp-content/uploads/2020/05/AN50-Spirulina-biomass_2018_02_02.pdf (accessed January 17, 2021).
H.H. Günther, F. Bergter, Bestimmung der Trockenmasse von Zellsuspensionen durch Extinktionsmessungen, Zeitschrift Für Allgemeine Mikrobiologie. 11 (1971) 191–197. https://doi.org/10.1002/jobm.3630110303.
A.L. Koch, Turbidity measurements of bacterial cultures in some available commercial instruments, Analytical Biochemistry. 38 (1970) 252–259. https://doi.org/10.1016/0003-2697(70)90174-0.
W.M. Sattley, M.T. Madigan, K.S. Bender, D.A. Stahl, D.H. Buckley, Brock Biology of Microorganisms, Pearson, 2018.
Y. Huang, S. Qin, D. Zhang, L. Li, Y. Mu, Evaluation of Cell Disruption of Chlorella Vulgaris by Pressure-Assisted Ozonation and Ultrasonication, Energies. 9 (2016). https://doi.org/10.3390/en9030173.
F.A.C. Martins, J.P.J. Avila, Effects of the Reynolds number and structural damping on vortex-induced vibrations of elastically-mounted rigid cylinder, International Journal of Mechanical Sciences. 156 (2019) 235–249. https://doi.org/10.1016/j.ijmecsci.2019.03.024.
Lesson 10: Algae as a Source for Fuels, PennState College of Earth and Mineral Sciences. (n.d.). https://www.e-education.psu.edu/egee439/node/691 (accessed January 17, 2021).
R. Gardner, P. Peters, B. Peyton, K.E. Cooksey, Medium pH and nitrate concentration effects on accumulation of triacylglycerol in two members of the chlorophyta, Journal of Applied Phycology. 23 (2011) 1005–1016. https://doi.org/10.1007/s10811-010-9633-4.
Downloads
Published
How to Cite
Issue
Section
License
Copyright (c) 2021 Progress in Energy and Environment

This work is licensed under a Creative Commons Attribution-NonCommercial 4.0 International License.







