Bio-Mediated Production and Characterisation of Magnetic Nanoparticles using Fruit Peel Extract
DOI:
https://doi.org/10.37934/jrnn.1.1.5361Keywords:
Green Approach, Fruit Peel Extract, Fe3O4 Nanoparticles, Magnetic Nanofluids, Physicochemical PropertiesAbstract
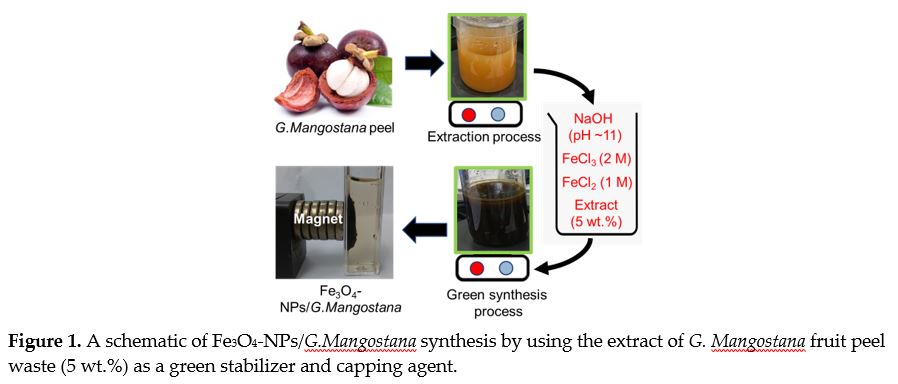
The overwhelming request for nanodevices and heat flow developments has led to consider magnetic Fe3O4 nanoparticles as a new dawn to the sophisticated nanotechnology in a sustainable manner. This research presented a facile production of Fe3O4 nanoparticles using co-precipitation method and the extract of Garcinia Mangostana fruit peel waste as a green stabilizer and capping agent. The X-ray powder diffraction (XRD) planes of the synthesized nanoparticles showed the formation of magnetite Fe3O4 nanoparticles with good crystallinity. Based on the image of field emission scanning electron microscope (FESEM), the diameter of the nanoparticles was estimated to be 69.14±2.87 nm as was coated by the extract. The Fe3O4 nanoparticles presented an acceptable magnetization value of 51.17 emu/g. From the analysis of Fourier-transform infrared spectroscopy (FTIR), the phenolic compounds and other functional groups of the extract had interactions with the Fe ions to successfully synthesize the nanoparticles. The green synthesized Fe3O4 nanofluids showed small hydrodynamic size of 145.80±3.14 and high zeta potential value of -30.5±1.82 mV. This study, thus, showed that the extract of Garcinia Mangostana fruit peel waste can serve as a bio-stabilizer and capping agent to enhance physiochemical properties and colloidal stability of the Fe3O4 nanofluids with an environmentally-friendly manner and low cost for modern applications.
Downloads
References
Kumar, A. and S. Subudhi, Preparation, characteristics, convection and applications of magnetic nanofluids: A review. Heat and Mass Transfer, 2018. 54(2): p. 241-265. doi: 10.1007/s00231-017-2114-4.
Felicia, L.J., S. Vinod, and J. Philip, Recent advances in magnetorheology of ferrofluids (magnetic nanofluids)—a critical review. J. Nanofluids, 2016. 5(1): p. 1-22. doi: 10.1166/jon.2016.1203.
Yew YP, Shameli K, Miyake M, Khairudin NBBA, Mohamad SEB, Naiki T, Lee KX, Green biosynthesis of superparamagnetic magnetite Fe3O4 nanoparticles and biomedical applications in targeted anticancer drug. Arab. J. Chem. 2020;13(1):2287-2308. doi: 10.1016/j.arabjc.2018.04.013.
Hedayatnasab Z, Abnisa F, Daud WMAW. Review on magnetic nanoparticles for magnetic nanofluid hyperthermia application. Mater. Des. 2017;123:174-196. doi: 10.1016/j.matdes.2017.03.036.
Yusefi, M., K. Shameli, and A.F. Jumaat, Preparation and Properties of Magnetic Iron Oxide Nanoparticles for Biomedical Applications: A Brief Review. Journal of Advanced Research in Materials Science, 2020. 75(1): p. 10-18. doi: 10.37934/arms.75.1.1018.
Yusefi, M., Shameli, K., Hedayatnasab, Z., Teow. S-Y., Ismail, U.N., Azlan, A.C., Ali, R.R., Green synthesis of Fe3O4 nanoparticles for hyperthermia, magnetic resonance imaging and 5-fluorouracil carrier in potential colorectal cancer treatment. Res. Chem. Intermed., 2021: p. 1-20. doi: 10.1007/s11164-020-04388-1.
Herlekar M, Barve S, Kumar R. Plant-mediated green synthesis of iron nanoparticles. J. Nanopart., 2014;2014. doi: 10.1155/2014/140614.
Magro M, Vianello F. Bare iron oxide nanoparticles: Surface tunability for biomedical, sensing and environmental applications. Nanomaterials, 2019;9(11):1608. doi: 10.3390/nano9111608..
Yusefi M, Shameli K, Ali RR, Pang S-W, Teow S-Y. Evaluating anticancer activity of plant-mediated synthesized iron oxide nanoparticles using Punica granatum fruit peel extract. J. Mol. Struct., 2020;1204:127539. doi: 10.1016/j.molstruc.2019.127539.
Kallumadil, M., Tada, M., Nakagawa, T., Abe. M., Southern, P., Pankhurst, Q.A., Suitability of commercial colloids for magnetic hyperthermia. J. Magn. Magn., 2009. 321(10): p. 1509-1513. doi: 10.1016/j.jmmm.2009.02.075
Ogholbeyg AB, Kianvash A, Hajalilou A, Abouzari-Lotf E, Zarebkohan A. Cytotoxicity characteristics of green assisted-synthesized superparamagnetic maghemite (?-Fe2O3) nanoparticles. J. Mater. Sci. Mater., Electron 2018;29(14):12135-12143. doi: 10.1007/s10854-018-9321-8.
Izadiyan Z, Shameli K, Miyake M, et al. Cytotoxicity assay of plant-mediated synthesized iron oxide nanoparticles using Juglans regia green husk extract. Arab. J. Chem., 2018. doi: 10.1016/j.arabjc.2018.02.019.
Tungmunnithum D, Thongboonyou A, Pholboon A, Yangsabai A. Flavonoids and other phenolic compounds from medicinal plants for pharmaceutical and medical aspects: An overview. Medicines, 2018;5(3):93. doi: 10.3390/medicines5030093.
Suttirak W, Manurakchinakorn S. In vitro antioxidant properties of mangosteen peel extract. J. Food Sci. Technol., 2014;51(12):3546-3558. doi: 10.1007/s13197-012-0887-5.
El-Faham S, Mohsen M, Sharaf A, Zaky A. Utilization of mango peels as a source of polyphenolic antioxidants. Curr. Sci. Int., 2016;5(04):529-542
Yew YP, Shameli K, Mohamad SEB, et al. Potential anticancer activity of protocatechuic acid loaded in montmorillonite/Fe3O4 nanocomposites stabilized by seaweed Kappaphycus alvarezii. Int. J. Pharm., 2019;572:118743. doi: 10.1016/j.ijpharm.2019.118743.
Xin Lee K, Shameli K, Miyake M, et al. Green synthesis of gold nanoparticles using aqueous extract of Garcinia mangostana fruit peels. J. Nanomater., 2016;2016. doi: 10.1155/2016/8489094.
Lee KX, Shameli K, Mohamad SE, et al. Bio-Mediated Synthesis and characterisation of silver nanocarrier, and its potent anticancer action. Nanomaterials, 2019;9(10):1423. doi: 10.3390/nano9101423
Holzwarth U, Gibson N. The Scherrer equation versus the'Debye-Scherrer equation'. Nat. Nanotechnol., 2011;6(9):534-534. doi: 10.1038/nnano.2011.145
Kroon R. Nanoscience and the Scherrer equation versus the'Scherrer-Gottingen equation'. S. Afr. J. Sci. 2013;109(5-6):01-02. doi: 10.1590/sajs.2013/a0019.
Lassoued, A., Dkhil, B., Gadri, A., Ammar, S. Control of the shape and size of iron oxide (?-Fe2O3) nanoparticles synthesized through the chemical precipitation method. Results Phys., 2017. 7: p. 3007-3015. doi: 10.1016/j.rinp.2017.07.066.
Yang J, Kim J, Lee J, et al. Inverted hysteresis loops observed in a randomly distributed cobalt nanoparticle system. Phys. Rev. B, 2008;78(9):094415. doi: 10.1103/PhysRevB.78.094415.
Sathishkumar G, Logeshwaran V, Sarathbabu S, et al. Green synthesis of magnetic Fe3O4 nanoparticles using Couroupita guianensis Aubl. fruit extract for their antibacterial and cytotoxicity activities. Artif. Cell Nanomed., 2018;46(3):589-598. doi: 10.1080/21691401.2017.1332635.
Testa-Anta M, Ramos-Docampo MA, Comesaña-Hermo M, Rivas-Murias B, Salgueiriño V. Raman spectroscopy to unravel the magnetic properties of iron oxide nanocrystals for bio-related applications. Nanoscale Advances., 2019;1(6):2086-2103. doi:10.1039/C9NA00064J.