Innovative Nanodelivery Systems Transform Tuberculosis Treatment with Pyrazinamide-loaded Chitosan Nanoparticles
DOI:
https://doi.org/10.37934/armne.19.1.2237Keywords:
Chitosan nanoparticle, ethambutol, tuberculosis, nanodelivery formulationAbstract
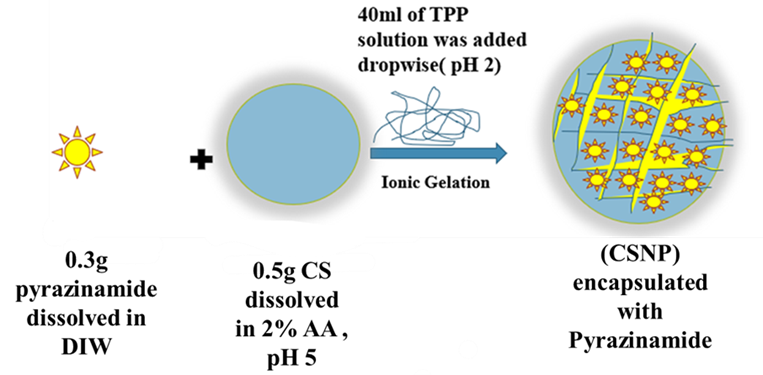
Tuberculosis (TB) stands as an enduring and formidable global health challenge, necessitating innovative methodologies to optimize therapeutic outcomes. Within this context, nanotechnology emerges as a promising modality to augment TB therapy by enabling targeted drug delivery of anti-TB agents to infect cells, thereby maximizing therapeutic efficacy while mitigating adverse effects. This study focalizes on the synthesis of chitosan nanoparticles (CSNP) as a nanocarrier for the concurrent delivery of Pyrazinamide (PYR), a pivotal antibiotic in the first-line treatment of TB. The primary objective is to overcome challenges related to the limited solubility and bioavailability of these drugs, which hinder their effectiveness and pose toxicity risks. The encapsulation of PYR within CS proves advantageous, enhancing solubility, bioavailability, and targeted drug delivery. The resulting nanocarrier, denoted as CS-PYR, is prepared through ionic gelation, involving cross-linking chitosan-loaded anti-TB carriers with tripolyphosphate to achieve nanoscale dimensions. Various analytical techniques, including XRD, FTIR, TGA, TEM, FESEM, DLS, UV-vis spectroscopy, antibacterial assays, and cytotoxic MTT assays, are utilized for a comprehensive investigation. The spherical morphology of PYR-CSNP, with an average diameter of 60 to 100 nm, is confirmed by FESEM, TEM, and DLS analyses. FTIR and XRD analyses validate the successful fabrication and multifunctional properties of PYR incorporated into the CSNP matrix. The drug-loaded CSNP exhibits improved thermal stability, displaying decomposition between 200-400°C, indicating higher temperature resistance and greater weight loss compared to the CSNP carrier due to PYR inclusion. Drug release studies show sustained release behaviour, with 90-99% release in an acidic (pH 4.8) buffer solution over seven days, following a pseudo-second-order model. Moreover, the CS-PYR nanocarrier demonstrates superior efficacy against Gram-negative and Gram-positive bacteria, attributed to drug intercalation resulting in larger inhibition zones. Cytotoxicity assessments for CS-PYR indicate 80% cell viability in MRC5 cells, underscoring the nanocarrier's potential as a safe and effective TB treatment delivery system without adversely affecting normal cells. These findings position CS-PYR as a promising nanodelivery system for TB therapy, offering enhanced precision in drug delivery and safety.